Links from our newsletters
You are interested in the studies we introduced in our latest newsletters?
Here we linked them for you.
"Autoimmune Disease - Full Development Support"

Peripheral target detection far from the site of action
Different roads of administration - like enteric-coated tablets, nasal spray, or intramuscular injections -are formulated to ensure adequate drug concentrations at the sites of action.
However, access to these sites for pharmacological sampling is often limited.
Matrices like CSF (cerebrospinal fluid), BALF (bronchoalveolar lavage fluid), or tumor biopsies do not only require invasive procedures burdening the patient, but also result in very limited sample volumes.
Blood samples are more easily accessible, but do not necessarily correlate with drug exposure at the site of action, since only low levels may leak into the periphery.
Endogenous levels are often below the sensitivity limits of conventional LBAs (ligand binding assays). Here, our Immuno-PCR technology Imperacer® allows to detect smallest drug traces even far from the difficult‐to‐access compartment.

Case study: GM-CSF quantification below 1 pg/ml
Human GM-CSF (Granulocyte Macrophage Colony Stimulation Factor) is secreted by various cell types of the immune system and believed to play a role in the development of autoimmune inflammatory diseases like Multiple Sclerosis, rheumatoid Arthritis or atopic Asthma.
Recombinant GM-CSF is applied as an immune modulating drug and clinically used to prevent neutropenia in cancer and AIDS patients undergoing therapy.
Chimera developed an immunoassay for the ultra sensitive quantification of human GM-CSF in the range of 0.0122 – 21,900 pg/ml, suitable for GLP-compliant clinical sample testing support.
"Autoimmune Disease - Full Development Support"

Ultra sensitive Biomarker Detection
Autoimmune disorders are complex chronic diseases, with inflammatory processes directed against the immune system. Inflammation is either directed against a specific organ as in Multiple Sclerosis or Crohn’s Disease, or affects the entire system in systemic autoimmune diseases such as Rheumatoid Arthritis and Psoriasis.
With the goal to specifically alter the immune regulation and activity in these diseases, the use of biologic therapies for the management of autoimmune diseases is rapidly expanding, due to beneficial characteristics of biologics. Most biologic therapy targets are cytokines, B cells, and co-stimulation molecules.
Good efficacy and safety profiles of these drugs as well as a better understanding of the targets are key to provide lasting therapeutic success.
Chimera Biotec is a GLP compliant CRO supporting customers from around the globe in their drug development efforts to fight autoimmune diseases. With more than 20 years of experience in tailoring ligand-binding assays to nearly any study requirement, we support you from tox to clinic providing quality driven and reliable data from nearly any study matrix (Serum, Plasma, Tissue, Synovial fluid, BALF, CSF, and more…).
From low abundance cytokines in healthy individuals to cytokine boost in autoimmune reactions

Case study: IL-2 and IL-6 clinical biomarker support
Multi-analyte cytokine testing from healthy volunteers and two inflammatory disease populations required sensitivities in the single digit pg/mL range for targeting IL-2 and IL-6 in the healthy population, and a 4 log assay range to monitor the interleukin levels in the diseased population. One assay was developed to cover the required assay range from less than 5 µl of serum sample.
Case study: IL-1a, IL-1b and IL-20 biomarker support in synovial fluid
IL-20 elevated pro-inflammatory and angiogenic activity is associated with e.g. psoriasis, rheumatoid arthritis, osteoarthritis. Here, we were able to quantify IL-20 levels from synovial fluid of patiens with Osteoarthritis or Rheumatoid Arthiritis.

The broad assay range allowed detection between 0.32 - 5,000 pg/ml.
In another study, Chimera successfully validated Imperacer® methods for the quantification of IL-1 alpha and IL-1 beta in human synovial fluid.
Lower limits of quantification were 0.3 pg/ml for IL-1a and 0.12 pg/ml for IL-1b. In both cases, the minimal sample volume required was 7 µl.
Due to its assay range of more than 4 log units, the Immuno-PCR platform Imperacer® was best suited for bioanalytical sample support,
balancing the requirements of a wide assay range in combination with limited sample availability.
"Simoa® Biomarker Testing Service"

Ultra sensitive Biomarker Detection
For the identification of the next promising drug candidate or early COVID-19 severity prediction, the demand for reliable ultra sensitive biomarker detection is increasing extensively.
The Simoa® platform combines single molecule analysis with a digital ELISA readout, enabling the recognition of low expressed biomarker.
As official Quanterix Partner, Chimera Biotec offers full Simoa® testing service for all your bioanalytical needs.
With more than 20 years of experience in ultra sensitive immunoassays, our team is ready to take your research to the next level.
Ready to use off-the-shelf Simoa® kits are available for various biomarkers.
Chimera´s Simoa® Expertise
- Biomarker support
- Kit-based services
- Bioanalystical services
- Adapting your in-house assays
- Assay development based on Quanterix homebrew kit
- Assay validation
- Matching bioanalytical service on all our platforms, including Imperacer®, MSD™and ELISA
"Your ultra sensitive GLP/GCP lab"

Study Needs | We Provide |
| GLP/GCP | FDA and EMA compliance |
Sensitivity | Detect sub pg/ml in matrix Low-dosing, low-level biomarkers |
| Assay range | 3-6 log units (fg-µg/ml) One assay from TOX to clinic |
| Sample volume | Immunoassay microsampling Below 10 µl, saliva, BALF, lysates and more |
Overcoming challenges
Working with rare matrices or invasive methods requires microsampling - we can work with sample volumes down to 1 µl.
Discover our AnySource® Dilution Technology. We can make more out of small sample volumes, while keeping ultra sensitive detection by exponential signal amplification via Imperacer®.
"Early Phase PK Data - Accerlerate your drug pipeline"

PK data for low-dosing drug candidates
Modern highly potent large-molecule drug candidates require careful safety and efficacy considerations. In immunotherapies, antibody-based T-cell therapies have been associated with TOX profiles and cytokine release syndrome.
This has been well known in immunotherapies, where antibody-based T-cell therapies have been associated with TOX profiles and cytokine release syndrome.
In consequence, dose escalation starts with extremely low dosing, but and may remain close to below the LLOQ in many early bioanalytical clinical study support opposite to high concentrations in preclinical TOX studies.
Therefore, PK supporting sample testing ideally combines
(i) detection of very low target concentrations (single digit fg or pg/mL) with
(ii) broad concentration range (covering TOX and low-dosing) all in one assay.

We provide meaningful PK data at your needed sensitivity, e.g. when drug leaks from the site of action into the periphery and needs to be quantified from blood samples.
Combine excellent sensitivities & broad quantitative assay range
There is no need to validate various immunoassays. Chimera supports your whole biologics PK study in one assay method on Imperacer®.

Sample testing with validated methods from a single assay format.
Quantifying low-dosed drug concentrations, we provide PK data from early phase of your clinical dose escalation trials under GCP.
Using our AnySource® Dilution Technology to reduce matrix effects, we can make more out of small sample volumes, but keep ultra sensitive detection via Immuno-PCR-based signal generation.
This strategy helps with clinical study design and patient enrolment and accelerates the drug development process to quickly move forward to regulatory filing.
"Vaccination - No Cold Chain, No Needles"

Thermostable Vaccines
Maintenance of the cold chain is mandatory to ensure the stability of fragile biological products. Global vaccine distribution is hampered, since not for every region a continuous storage cold chain can be assured.
In addition, needle-free, thermostable vaccines can significantly improve global access and acceptance to vaccination programs.
Being partner in a European research consortium (MACIVIVA), Chimera Biotec has used its proprietary Immuno-PCR platform Imperacer® to support the development of needle-free, thermostable solid vaccines.
Chimera’s immune-analytical expertise resulting from these research and development efforts can be applied to support other vaccination programs as well. For example, to overcome analytical challenges resulting from comparable site-of-action biological test specimen.
In case of indications affecting the lung, such as Covid-19, antibody-, but also biomarkers or drug-levels can be measured from lung samples, such as
♦ Sputum,
♦ Bronchoalveolar lavage fluid (BALF)
♦ Exhaled breath condensate (EBC)
♦ and more
Mucosal Vaccination
Viral infection can exploit weaknesses of the immune system or directly attack it. Classic approaches focus on neutralizing antibodies in the bloodstream, However, with viruses like HIV, once the virus has infected immune cells, it is already too late.
Mucosal vaccination approaches aim to block early absorption events of mucosal transmitted viruses and thus inhibit infection by blocking transport of virus particle through mucosal tissue into the lymphatic system. Antibody response blocking the needed receptor interactions leads to protection.
The predominant antibody form in mucosal tissue is IgA, able to block proteins that are needed for transport, however IgG response plays a significant part in mucosa as well. Therefore, vaccination status must be detected in mucosal tissue where antibody concentration is 100 times lower and samples are harder to take in a standardized way.
The resulting bioanalytical challenges exceed ELISA possibilities:
♦ Mucosal antibody concentration is 100-fold lower compared to blood
♦ Mucosal washes hold even lower concentrations
♦ Detection from limited sample volume
♦ Measuring multiple analytes in parallel
♦ Discriminate vaccine- as well as total antibody response from various matrices e.g. mucosal tissue and blood samples
♦ Combine excellent sensitivity and broad assay range
Chimera mastered these challenges with the development of ultra sensitive Immunoassays, capable of measuring vaccine specific mucosal antibodies and total immune response at the needed sensitivities and concentration ranges from limited sample volumes of mucosal washes.
Our Imperacer® assays allow parallel double determination of multiple analytes from less than 10 µl sample volume - in this case 6 analytes from 8 µl.
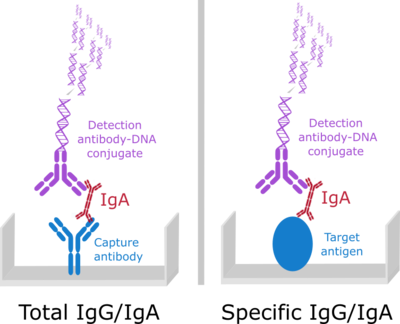
Combining bridging and sandwich assay formats, custom developed for each analytical task on the ultra sensitive Immuno-PCR based Imperacer platform, we supported proof of concept preclinical studies and a clinical trial on HIV-1 mucosal virosome vaccination.
Virosomes are efficient antigen delivery vehicles. Their empty lumen lacks nucleic acid, making them non-infectious.
With the help of Chimera’s state-of-the art immunoassay analytics, virosomes displaying a particular HIV antigen structure, have been shown to increase mucosal antibodies blocking early transmission events. Since respiratory, genital and gastrointestinal mucosa are interconnected, NK cells, T and B lymphocytes can migrate and seed to distant mucosal tissue.
Thus, a more robust genital and intestinal immune response can be elicited by two defense lines:
♦ 1st - Antigen-specific immune cells provide mucosal protection
♦ 2nd - Generation of blood antibodies
Read more
Aided by Chimera’s Immunoassay monitoring of mucosal as well as blood level antibody response, the study shows a strong vaccine-triggered inhibition in HIV-1 transcytosis by increased vaginal HIV antigen-specific IgG and IgA antibodies. Despite low antibody contents, as compared to serums, vaginal secretions of vaccinated women have been demonstrated to harbor antiviral activity.
Amacker et al. (2020) New GMP manufacturing processes to obtain thermostable HIV-1 gp41 virosomes under solid forms for various mucosal vaccination routes
Leroux-Roel et al. (2013) Randomized Phase I: Safety, Immunogenicity and Mucosal Antiviral Activity in Young Healthy Women Vaccinated with HIV-1 Gp41 P1 Peptide on Virosomes
"The Power of Sample Dilution"
Limited Sample Volume
While microsampling had been a topic mostly discussed in pre-clinical studies with small animals or rare matrices, nowadays many people have experienced microsampling themselves - for example when they had to do a Covid-19 test.
Specimen collected may be
- Nasopharyngeal or oropharyngeal mucus
- Nasal wash or aspirate
- Saliva
Other rare matrices often require even more invasive procedures:
- Cerebrospinal fluid - Lumbar punction
- Aqueous humor - Syringe injection
- Bronchoalveolar lavage fluid - Tracheal washes
Overcoming challenges
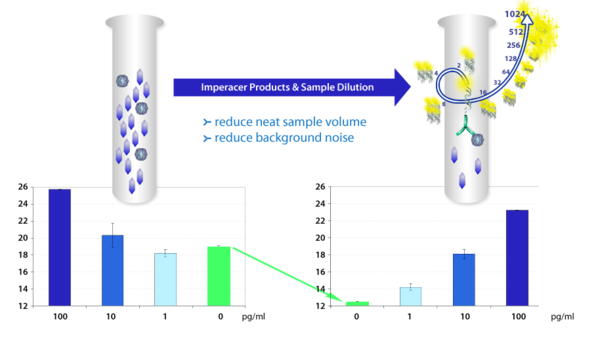
Using our AnySource® Dilution Technology, we can make more out of small sample volumes. Even from volumes down to 1 µl, we can run multiple assays whilst keeping ultra sensitive detection by exponential signal amplification via Imperacer®.
"GLP / GCP Bioanalytical Service"
Overcoming challenges
Working with rare matrices or invasive methods requires microsampling - we can work with sample volumes down to 1 µl.
Discover our AnySource® Dilution Technology. We can make more out of small sample volumes, while keeping ultra sensitive detection by exponential signal amplification via Imperacer®.
"Cytokine Storm Biomarkers"

Inflammatory biomarkers
Several studies investigating severe COVID-19 cases, cite a hyperactivation of the inflammatory response, leading to a cytokine release syndrom, also called cytokine storm.
Many reports show elevated levels of IL-1, IL-6 , TNF-α and C-reactive protein (CRP), additionally, a protective effect for blood type 0 is discussed.
Read more in the following studies.
Furthermore, we provide exquisite bioanalytical biomarker services on different platforms.
Find suited Imperacer®, Simoa®, MSD™ or ELISA biomaker kits or request your custom-designed assay here.
Levi, M. et al. (2020) Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 7(6): e438–e440.
Kermali, M. et al. (2020) The role of biomarkers in diagnosis of COVID-19 – A systematic review. Life Sci 117788.
Chen, L. et al. (2020) Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Chinese J Tuberculosis Respir Dis 43(0):E005
Guan, W. et al. (2020) Clinical Characteristics of Coronavirus Disease 2019 in China. NEJM. 382: 18
Wang, W. et al. (2020) The definition and risks of Cytokine Release Syndrome-Like in 11 COVID-19-Infected Pneumonia critically ill patients: Disease Characteristics and Retrospective Analysis. medRxiv
"20 Years Immunoassay Expertise"
Chimera Biotec Celebrates!
Starting in 2000, with our proprietary Immuno-PCR platform Imperacer®, Chimera has specialized in ultra sensitive GLP/GCP bioanalytical support for all phases of drug discovery.

Get your assay! Get excellence!
♦ GLP / GCP test facility
♦ Non-regulated, preclinical & clinical support
♦ Compliant with FDA & EMA guidances
♦ Ultra sensitive immunoassay platforms
♦ Assay feasibility
♦ Full assay devevlopment services
♦ Kit-based services
Ever wondered how
companies come up
with their names?
This is our story:

A Chimera is a mixed being in Greek mythology:
“She was of divine stock, not of men, in the fore part a lion, in the hinder a serpent, and in the midst a goat, breathing forth in terrible wise the might of blazing fire.” (Homer, Iliad 6,180 ff.)
Our Chimera is the Immuno-PCR platform Imperacer®, in which antibody-DNA conjugates combine ELISA-type immunoassay setup with exponential PCR read-out:
“She was of ultra sensitivity, in the fore part antibody, in the hinder DNA, enabling forth in outstanding sensitive wise the might of groundbreaking science.”
Bring together Imperacer®, AnySource® and Chimera´s 20 years of experience in beyond ELISA sensitive immunoassays, combining ultra sensitivity, broad assay range and minimal sample volume requirement.
That´s why Chimera Biotec: We provide optimal assay development for your target and bioanalysis under GLP/GCP regulations.
"Bispecific T Cell Engager - Their Role in Immuno-Oncology"

Here, Ma et al. (2020) describe T cell engagers (TCEs) binding to the tumor marker CEA (serum carcinoembryonic antigen) on cancer cells
and CD3 on T cells in the tumor compartment to form bivalent TCE ternary complex (biTTC).
They use a quantitative systems pharmacology (QPS) model to explore TCE efficiency, identify potential biomarkers
and can even predict patient-specific response to TCE treatment.

We routinely develop and validate ultra sensitive PK assays for GLP/GCP regulated bioanalytical sample testing support of bispecific antibody-based therapies, such as TCRs, for treatment of various malignancies.
Chimera´s AnySource® sample dilution minimizes matrix effects, enabling therapeutic antibodies target detection in the presence of ~ 1,000,000-fold excess of endogenous antibodies.
We offer complete service packages (PK/PD, Immunogenicity, Biomarker) across multiple platforms.
"Neurodegenerative Diseases - Bioanalytical Solutions"

Here, Yoo et al. (2020) suggest soluble Aβ*56 and AβO as potential biomarkers for AD in nasal discharge.
While increased levels in Aβ*56 alone can indicate mild AD, additionally increased AβO levels give hint to a moderate stage.
This suggests soluble Aβ*56 and AβO as potential biomarkers for AD in nasal discharge.

To address the low oral bioavailability and side effects of levodopa, Arisoy et al. (2020) used nano-sized drug carriers for nose to brain delivery.
The levodopa-coated biocompatible nanoparticles prolonged release up to 9h and improved locomotor activity in Parkinson´s Diesease model in mice.
Please feel free to contact Chimera Biotec’s team of dedicated scientists for more in-depth information. We are looking forward to provide you with the immunoassay to exceed your highest expectations.