Ultra Sensitive Support meets your Regulatory Requirements
As a service provider, we have developed technical capabilities on several ultra sensitive platforms, starting with high sensitivity (HS) ELISA, Simoa® technology from Quanterix, MSD™ (Mesoscale Discovery) as well as our own Immuno-PCR (IPCR) platform technology, Imperacer®. We deliver immunoassays to fulfill even your most challenging study requirements.
Our laboratory is GLP-certified and offers immunoassay services under the regulation of your choice:
♦ GLP
♦ GCP
♦ non-GxP
with compliance to FDA and EMA.
From internal decision making to GLP/GCP regulated studies
We make sure to deliver immunoassay sensitivity under the regulatory regime you need.
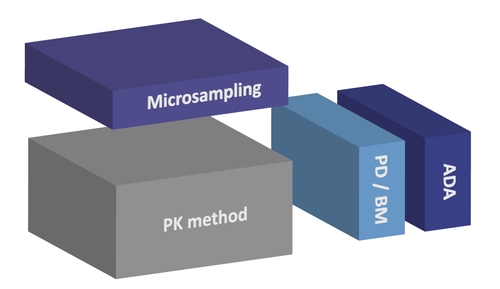
The development of biotherapeutic drugs is a very complex process which presents many challenges that may change over the curse of the study. Regulatory requirements which may change over the different phases of a compound’s pharmaceutical development. Chimera Biotec is specialized in overcoming these challenges, regarding to e.g.:
♦ bioanalytical support
♦ requirements for sensitivity
♦ sample volume availability
♦ overall assay performance and
♦ quantification range
Relying on our cumulative expert knowledge and laboratory capabilities, we deliver top-quality bioanalysis on-time.
Bioanalysis
Your study samples will be stored, handled, analyzed, reported and archived with greatest care and swift turn around:
♦ Ultra sensitive ligand-binding assay sample testing
♦ GLP / GCP regulated bioanalytical support or exploratory sample testing
♦ Sample logistics and data reconciliation services supporting multi-center clinical trials
♦ Sample storage in 24/7 monitored environment
Bioanalytical support for biotherapeutics throughout all phases of drug development.
From high to extreme low dosing, we provide regulated sample testing using validated methods, based on the same assay. Our immunoassay validation is carried out as detailed by the Federal Drug Association (FDA) and European Medicines Agency (EMA) guidelines for Bioanalytical Method Validation.
♦ Exploratory
♦ TOX
♦ Dose escalation
♦ Pre-clinical
♦ Clinical