Taking responsibility for bioanalytical data begins with validation
Our expert scientific team will ensure that your needs for bioanalytical support and regulatory guidance are perfectly met.
Specific wishes?
We are dedicated to include all of your required parameters and adapt our procedures to your needs.

Whether you are looking for
♦ non-regulated discovery phase support
♦ GCP clinical phase support
♦ GLP-regime for IND-enabling TOX
we will make sure that immunoassay method validation is custom designed according to your expectations of bioanalytical support and if required in
♦ compliance to FDA and EMA guidances.
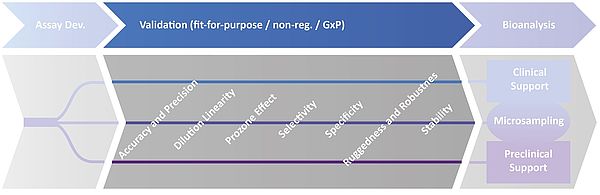
Validation Parameter
Depending on your study design, we are happy to ex- or include validation/ qualification parameters of your choice, e.g.:
♦ Calibration model (calibration curve)
♦ Sensitivity (LLOQ) and quantification range
♦ Intra-assay precision and accuracy
♦ Inter-assay precision and accuracy
♦ Dilution Linearity
♦ Prozone / Hook Effect
♦ Selectivity (matrix interference)
♦ Specificity (target interference)
♦ Ruggedness and Robustness
♦ Parallelism
♦ Freeze-thaw stability
♦ Short-term stability (Bench-top stability)
♦ Long-term stability (Frozen storage stability)
Check out the other steps or browse our capabilities
Chimera Biotec:
Your GLP-certifed CRO for ultra sensitive & technically demanding immunoassays since 2000
Our expertise in the development and validation of ultra sensitive immunoassays allows us to validate methods on ultra sensitive technology platforms in accordance with the general guidance documents.