Your study needs define our assays
We tailor your customized assay to your study or use already available kits.
As your single point of contact, your dedicated project manager at Chimera ensures that your scientific input guides our assay development towards the optimal assay procedure on the ideal platform.
Our laboratory is GLP-certified and offers immunoassay services under the regulation of your choice:
♦ GLP
♦ GCP
♦ non-GxP
with compliance to FDA and EMA.
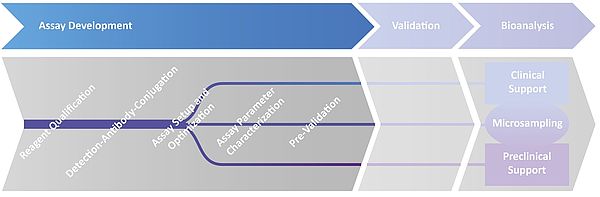
Assay Development
1. Technology evaluation:
Keep working on your preferred in-house platform and only outsource case studies where ultra sensitivity or related criteria is a must. We have the scientific expertise and technical capabilities to facilitate your choice of ideal platform technology based on your pharmacological requirements.
2. Reagent Qualification:
From your proprietary reagents or commercially available materials, we select those which are most suitable to fulfill the study requirements.
Having several candidates in mind? We quickly perform feasibility studies and you decide with which candidates you want to proceed.
3. Detection-Antibody conjugation:
Depending on the preferred immunoassay platform, we synthesize or select the optimal detection-conjugate (e.g. antibody-DNA conjugate, antibody-enzyme conjugate, antibody-fluorophor conjugate, etc.).

4. Assay setup and optimization:
Once the core assay reagents are in place, the assay is developed in agreement with bioanalytical guidance documents.
Thanks to our AnySource® sample dilution, we can adapt an assay to multiple (e.g. animal, artificial, human)
or challenging matrices (e.g. CSF, synovial fluid, aqueous humor, etc.).
Furthermore, we optimize required sample volume (e.g. for microsampling or rare matrix study support), and increase selectivity while reducing background noise.
5. Assay performance:
Including qualification/ pre-validation, the developed assay(s) are converted into bioanalytical method(s) ready for method validation.
Check out the next steps or browse our capabilities
Chimera Biotec:
Your GLP-certifed CRO for ultra sensitive & technically demanding immunoassays since 2000
For over 20 years, we have specialized in the development of immunoassays with highest demand on bioanalytical quality for biologics, biosimilars and biomarkers. Our long standing know how in beyond ELISA sensitive immunoassays enables us to make the most out of any immunoassay platform.